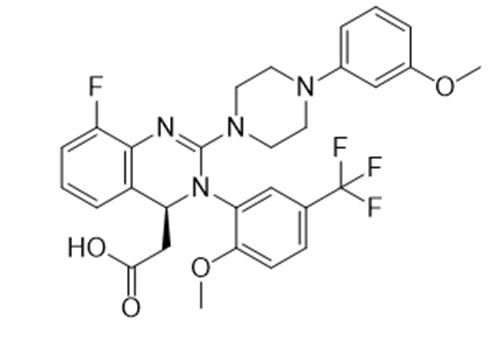
Letermovir
Product name: LeterMovir
Domestic registration status: Y20240000270 A
International registration: DMF in preparation
CAS No.: 917389-32-3
Molecular Formula: C29H28F4N4O4
Molecular weight: 572.55
EINECS No.: 200-001-8
Boiling point: 706.5±70.0°C(Predicted)
Density: 1.37±0.1g/cm3(Predicted)
Storage condition: Sealedindry,Storeinfreezer,under-20°C
Solubility: ≥57.3mg/mL in DMSO; insoluble in ethanol; insoluble in water
Form: solid
Acidity coefficient: (pKa)4.00±0.10(Predicted)
Color: beige to yellow
Application: Anti-cytomegalovirus drug, a novel CMV inhibitor, targeting viral telomerase to inhibit its activity.
Product specification: 1kg/barrel, 2kg/barrel, 5kg/barrel, 10kg/barrel, 15kg/barrel, 20kg/barrel, 25kg/barrel, can be separately sub-packaged according to customers' requirements.
Price: Price is for reference only, call us for one-to-one quotation.
Advantage: Source factory direct supply, high quality products, reasonable price, fast delivery and attentive after-sales service as the main advantage, support customized!
Advantageous products