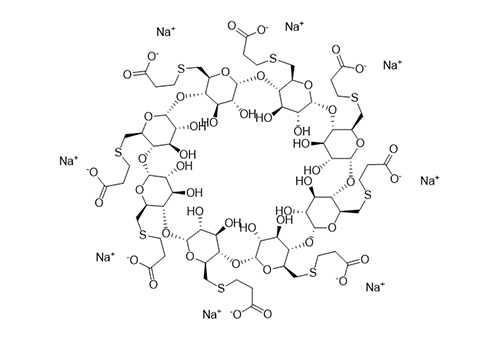
Sugammadex sodium
Product name:Sugammadex sodium
Main products: Vonorazan fumarate and intermediates, Dagliflozin and intermediates, Clevoborol and intermediates, Letemovir and intermediates, Mildronate, veterinary products (intermediates of Floreana, Aflana and Sarolana, etc.), Ropivacaine Hydrochloride and intermediates, local anesthesia series (Lidocaine, Lidocaine, Proparacaine, Proparacaine Hydrochloride, Bupivacaine Hydrochloride, etc.), covering local anesthesia drugs, local anesthesia drugs and intermediates, and covering local anesthetic drugs, and covering local anesthesia drugs, and covering local anesthesia drugs, and covering local anesthetic drugs. Our products cover the fields of local anesthetics, antiviral drugs, cardiovascular and cerebrovascular drugs, antitumor drugs, digestive drugs, diabetes drugs, veterinary drugs and so on. Our main advantages are high quality products, reasonable price, fast delivery and considerate after-sales service, and we support customized products.