
 Chenghui Shuangda
Chenghui Shuangda

【Breaking News】Shandong Chenghui Shuangda Pharmaceutical Co., Ltd.'s independently developed active pharmaceutical ingredient (API) Cliborol (Registration No.: Y20230000985) received approval from the National Medical Products Administration (NMPA) on August 22, 2025, for its “A” status (indicating approval for use in marketed formulations as an API/excipient/packaging material). This milestone signifies the API's official qualification for use in commercial drug products!
This breakthrough achievement fully demonstrates the company's professional strength in innovative drug R&D. From laboratory development to industrial-scale production, our stringent quality management system ensures products meet international standards. We now cordially invite pharmaceutical companies, R&D institutions, and industry partners to discuss collaboration opportunities. Together, let us seize this new opportunity and jointly advance the pharmaceutical industry!
Product Introduction
Product Name: Crisaborole
English Name: Crisaborole
CAS: 906673-24-3
Molecular Formula: C14H10BNO3
Structural Formula:
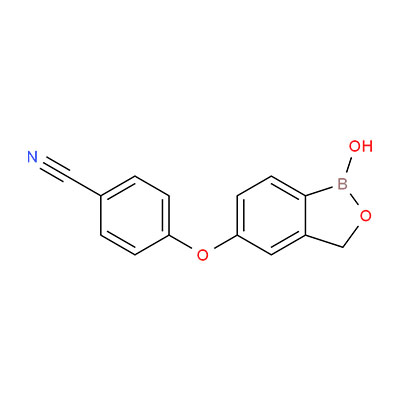
Application Overview: Crisaborole is a non-steroidal phosphodiesterase 4 (PDE4) inhibitor primarily indicated for the treatment of mild to moderate atopic dermatitis (eczema). By inhibiting key pathways in the inflammatory response, it effectively alleviates symptoms such as skin redness, swelling, and itching. Characterized by rapid onset and high safety profile, the approval of this active pharmaceutical ingredient (API) will provide a high-quality raw material for the R&D and production of related topical formulations (e.g., ointments, creams), thereby advancing innovative drug development in dermatology.