
 Chenghui Shuangda
Chenghui Shuangda

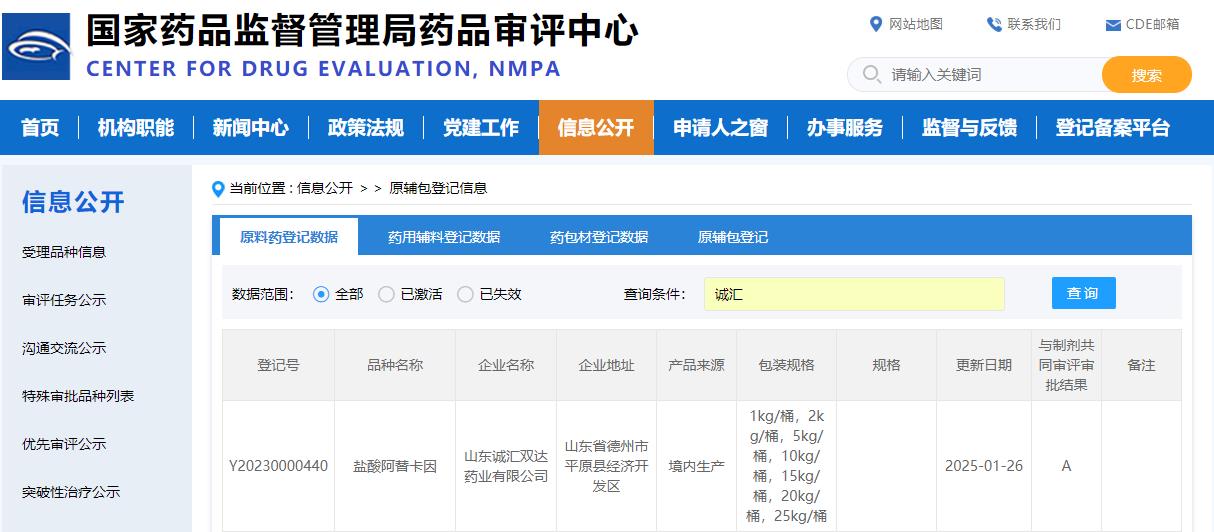
Recently, from the State Drug Administration Drug Evaluation Centre official website was informed that Shandong Chenghui Shuangda Pharmaceutical Co., Ltd. developed the API ativan hydrochloride (Registration No.: Y20230000440) on January 26, 2025 was approved for conversion to A (A indicates that the raw materials/excipients/packaging materials have been approved for use in the listed preparations). This good news not only reflects the excellent achievements of Chenghui Pharmaceutical in the field of API research and development, but also highlights the deep strength of our company in the production conversion and quality management, welcome new and old customers to negotiate and cooperate with us!
About the product:
Product name: Articaine hydrochloride
English name: Articaine Hydrochloride
CAS:23964-57-0
Molecular Formula: C13H21ClN2O3S
Application Overview:Articaine Hydrochloride is an amide local anaesthetic drug with dual effects of anaesthesia and analgesia, which is widely used in dental clinics, especially dental anaesthesia.